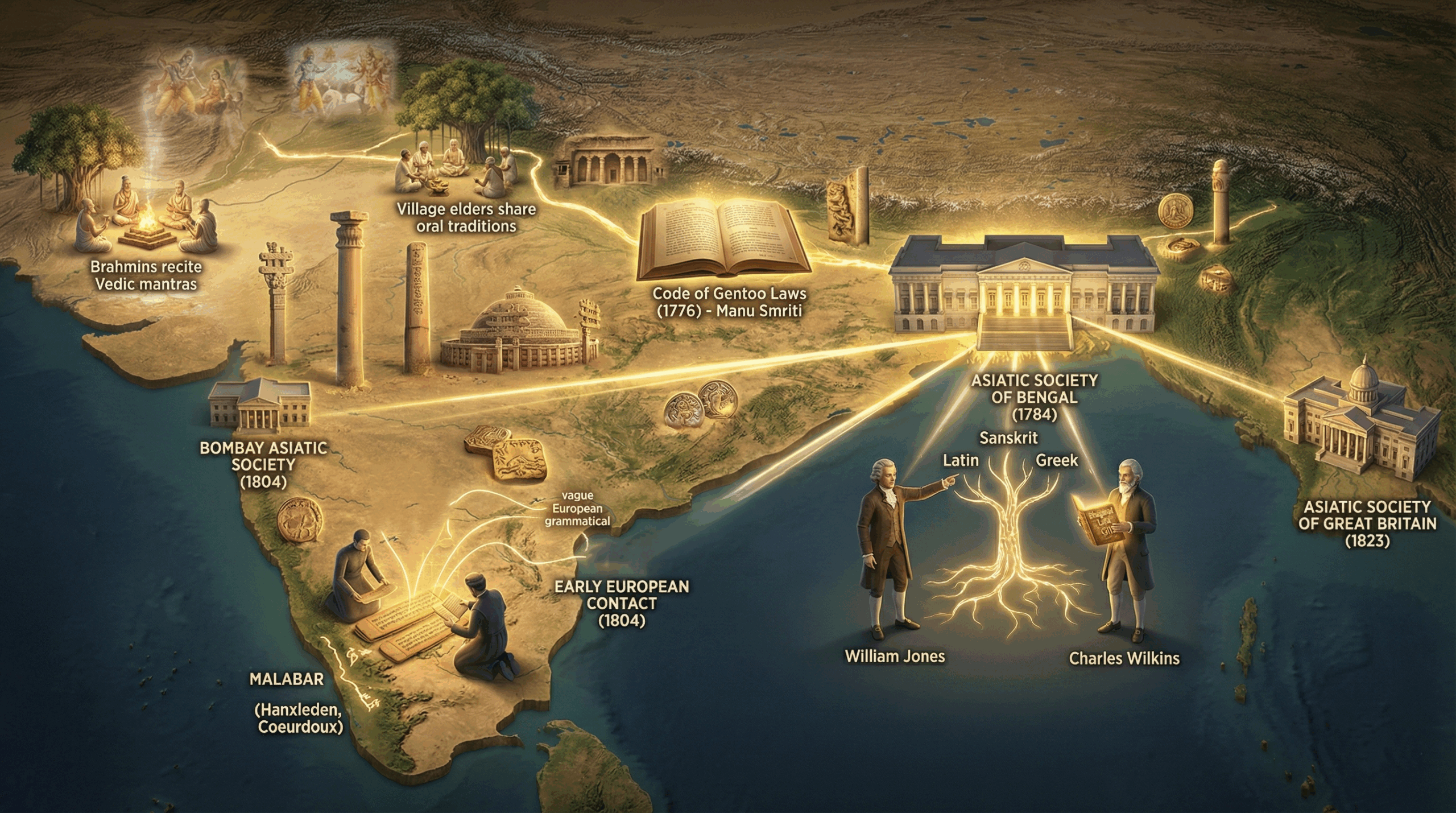
Every year on 26 January, India pauses to commemorate one of the most important milestones in its modern history the […]
Rethinking Cognitive Ageing: Biological Signatures Beyond Chronological Time
The human brain does not age along a single, uniform trajectory. Instead, brain ageing represents a spectrum of biological outcomes arising from cumulative interactions between genetic architecture, cellular metabolism, vascular integrity, immune regulation, lifestyle exposures, and environmental stressors. While many individuals retain functional neural networks and cognitive stability into later decades of life, others experience disproportionate neurobiological ageing that predisposes them to cognitive impairment and neurodegenerative disease, particularly Alzheimer’s disease and related dementias. Elucidating the determinants of this divergence is a central unresolved problem in ageing neuroscience.
Recent investigations led by King’s College London’s Centre for Healthy Brain Ageing have advanced the field by reframing brain ageing as a quantifiable, system-level biological phenotype rather than a purely chronological process. Through the integration of high-resolution neuroimaging and machine learning methodologies, this research has established “brain age” as a scalable biomarker capable of capturing cumulative neural vulnerability across distributed brain systems. Unlike traditional diagnostic markers that focus on region-specific pathology, brain age reflects global deviations in brain structure and organisation relative to normative ageing patterns.
This approach enables the identification of accelerated brain ageing at a stage when cognitive performance may still fall within clinically normal limits. By detecting these early deviations, researchers can distinguish physiological ageing from pathological processes years before overt neurodegenerative syndromes emerge. Importantly, this paradigm supports a shift from symptom-based classification toward biology-driven stratification of cognitive ageing risk.
From a translational perspective, the brain age framework offers a powerful tool for personalised risk assessment and precision prevention. By modelling individual brain ageing trajectories, it becomes possible to integrate neurobiological data with genetic, behavioural, and clinical variables, thereby refining predictions of future cognitive decline. Such advances have profound implications for the timing of intervention, participant selection in clinical trials, and the development of targeted strategies to preserve cognitive health across the lifespan.
Rethinking Brain Ageing: Normal versus Pathological Change
One of the central goals of brain ageing research is to distinguish between normal, age-related changes and those driven by neurodegenerative disease. Normal ageing is associated with gradual alterations in brain structure and function, including mild cortical thinning, reduced synaptic density, and subtle declines in processing speed. Importantly, these changes do not necessarily result in dementia or severe functional impairment.
In contrast, pathological ageing involves distinct biological processes, such as abnormal accumulation of amyloid-β plaques and tau neurofibrillary tangles, neuroinflammation, synaptic loss, and neuronal death. These changes disrupt neural networks critical for memory, attention, and executive function.
The challenge lies in detecting when the ageing brain deviates from a healthy trajectory. King’s College London researchers approach this problem by combining large-scale neuroimaging datasets with computational models capable of identifying subtle, system-level patterns that are invisible to conventional clinical assessment.
Brain Age as a Biomarker of Brain Health
Concept and Methodology
A major contribution of the Centre for Healthy Brain Ageing is the development and validation of “brain age” as a biomarker. Brain age is an estimate of how old a person’s brain appears based on structural features measured through magnetic resonance imaging (MRI). Using machine learning algorithms trained on thousands of brain scans, researchers identify patterns associated with chronological ageing and apply these models to new individuals.
The difference between predicted brain age and actual chronological age, often referred to as the “brain age gap” serves as an indicator of brain health. A positive gap, where the brain appears older than expected, suggests accelerated brain ageing.
Clinical and Biological Significance
Research has consistently shown that an older-appearing brain is not merely a statistical curiosity but carries meaningful clinical implications. Individuals with an increased brain age gap demonstrate:
- A higher risk of all-cause mortality.
- Poorer physical health indicators, such as reduced grip strength and slower walking speed.
- Greater cognitive decline over time
- Increased likelihood of progressing to dementia among those with existing memory impairments.
These findings position brain age as a powerful integrative biomarker that captures the cumulative impact of genetic vulnerability, disease burden, and lifestyle exposures on the brain.
Advanced Neuroimaging: Mapping the Ageing Brain
The accuracy of brain age estimation depends heavily on high-quality neuroimaging. King’s College London researchers employ a range of advanced MRI techniques to characterise brain structure and connectivity in detail.
Structural MRI enables precise measurement of cortical thickness, grey-matter volume, and regional atrophy patterns, particularly in areas vulnerable to Alzheimer’s disease such as the hippocampus and temporal lobes. Diffusion tensor imaging provides insights into white-matter integrity, revealing microstructural changes that affect communication between brain regions. Functional MRI contributes information about large-scale brain networks and their efficiency.
By integrating these imaging modalities, researchers gain a multidimensional view of brain ageing, allowing machine learning models to capture complex relationships between brain anatomy, function, and age.
Factors Influencing Brain Age
Negative Influences and Accelerated Ageing
King’s College London research highlights several factors associated with accelerated brain ageing. Psychiatric disorders, including schizophrenia and major depressive disorder, are consistently linked to older-appearing brains. These findings suggest that mental health conditions exert long-term effects on brain structure, potentially mediated by chronic stress, inflammation, or altered neurodevelopmental pathways.
Traumatic brain injury is another significant risk factor. Even a single injury can initiate processes that accelerate neurodegeneration, increasing vulnerability to later-life cognitive decline. Additionally, chronic positive energy balance, driven by poor diet and physical inactivity has been associated with adverse brain ageing profiles, reinforcing the connection between metabolic health and brain integrity.
Protective Factors and Cognitive Resilience
Importantly, brain ageing is not a fixed or inevitable process. The Centre’s research also identifies factors associated with a lower brain age and greater cognitive resilience. Higher levels of physical activity are linked to healthier brain ageing, likely through improved cerebrovascular function, reduced inflammation, and enhanced neuroplasticity.
Education appears to exert a protective effect, supporting the concept of cognitive reserve. Individuals with more years of education may better tolerate age-related brain changes without manifesting clinical symptoms. Engagement in mentally stimulating activities, such as playing musical instruments or practicing meditation has also been associated with younger-appearing brains, suggesting that lifelong cognitive engagement contributes to neural resilience.
The PROTECT Study: Real-World Insights into Brain Ageing
A cornerstone of King’s College London’s contribution to brain ageing research is its involvement in the UK-wide PROTECT study. This large, longitudinal online study follows adults aged 50 and above to investigate factors influencing cognitive health over time.
Recent findings from PROTECT revealed that cognitive performance declined more rapidly during the COVID-19 pandemic, particularly among individuals with pre-existing mild memory problems. These results highlight the sensitivity of brain health to external stressors, social isolation, and disruptions in healthcare access. They also underscore the importance of monitoring cognitive ageing in real-world contexts, where biological vulnerability interacts with environmental challenges.
Machine Learning and Individualised Brain Health
Traditional clinical approaches rely heavily on population averages, which can obscure individual variability. Machine learning allows researchers to move beyond this limitation by modelling brain ageing at the individual level. Algorithms can integrate neuroimaging, cognitive, genetic, and lifestyle data to generate personalised risk profiles.
This approach aligns with the broader shift toward precision medicine. Rather than waiting for symptoms to appear, clinicians may one day use brain age and related biomarkers to identify at-risk individuals decades earlier, enabling targeted prevention strategies.
Future Directions and Translational Potential
The Centre for Healthy Brain Ageing is now focused on translating these research insights into clinical and public health impact. Key future directions include:
Longitudinal studies designed to test whether lifestyle or medical interventions can slow or reverse accelerated brain ageing. These studies are essential to establish causality rather than mere association.
Investigation of brain-body interactions, particularly the role of peripheral organs such as the gut, liver, and kidneys in brain dysfunction during ageing. Growing evidence suggests systemic health strongly influences neurodegenerative risk.
Exploitation of large-scale datasets, including the UK Biobank, to explore genetic susceptibilities and individual variation in ageing trajectories. Such datasets allow unprecedented statistical power to identify subtle risk factors and protective mechanisms.
Conclusion
Research from King’s College London’s Centre for Healthy Brain Ageing is reshaping our understanding of how the brain ages and why some individuals are more vulnerable to cognitive decline than others. By combining advanced neuroimaging with machine learning, researchers have developed brain age as a robust biomarker that captures the cumulative effects of biology, lifestyle, and disease on brain health.
This work represents a critical step toward earlier diagnosis, personalised risk assessment, and ultimately prevention of neurodegenerative diseases. As the ageing population grows, such approaches will be essential for shifting dementia care from late-stage treatment to proactive brain health preservation.