Every year on 26 January, India pauses to commemorate one of the most important milestones in its modern history the […]
Innovative Approach Shows Promise in Revitalizing the Immune System
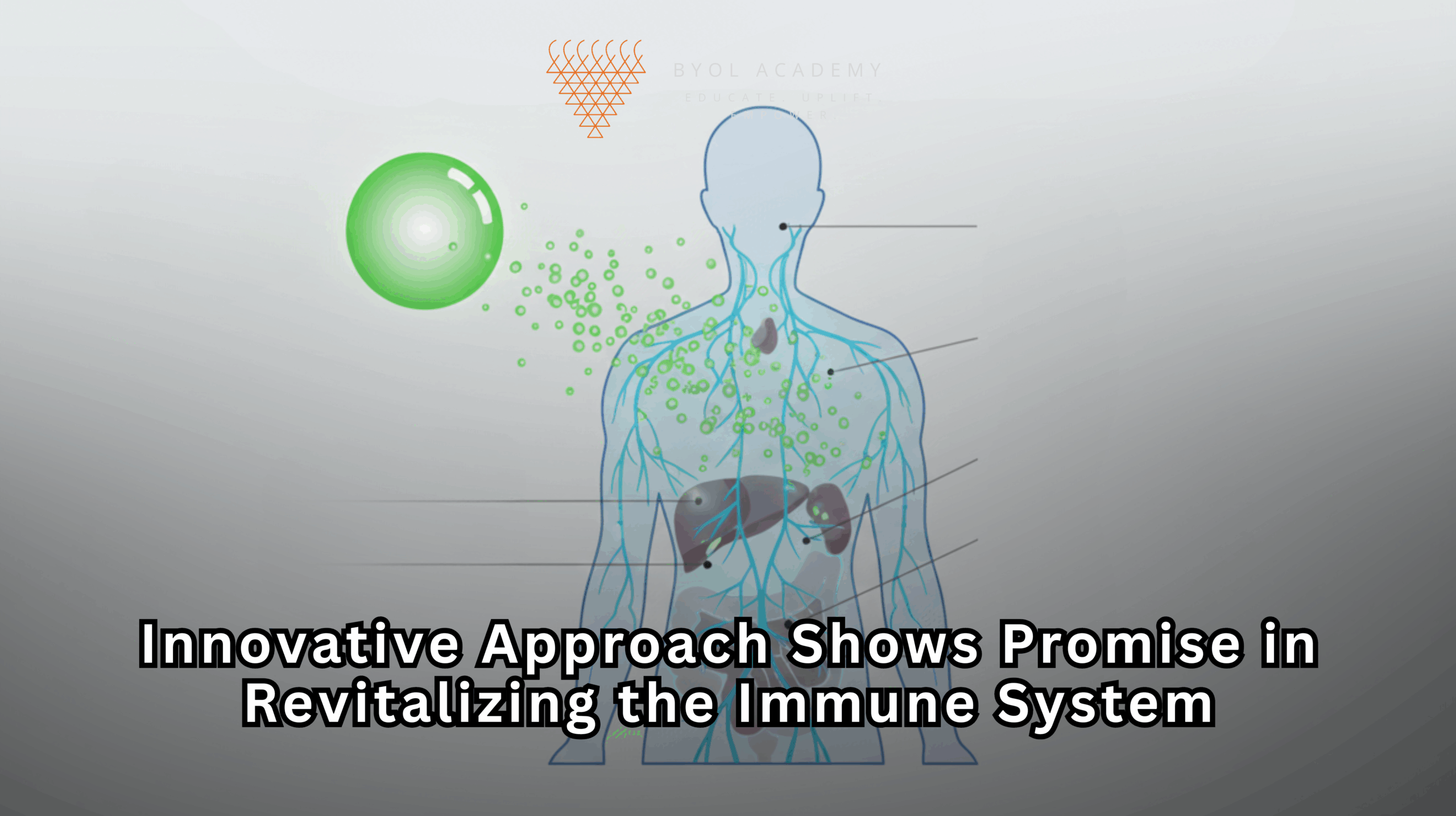
Aging is accompanied by a progressive decline in immune function, a phenomenon that significantly increases susceptibility to infections, diminishes vaccine efficacy, and impairs anti-tumor immunity. Central to this process is the reduction in T-cell populations, which play a critical role in adaptive immune responses. The thymus, a primary lymphoid organ located anterior to the heart, serves as the site for T-cell maturation and maintenance. Beginning in early adulthood, the thymus undergoes involution, characterized by a progressive loss of functional thymic tissue and a consequent decline in thymopoiesis. By the seventh decade of life, thymic activity is substantially diminished, contributing to age-associated immunosenescence.
In a recent study, investigators at the Massachusetts Institute of Technology (MIT) and the Broad Institute have developed a novel strategy to address this decline. By transiently programming hepatocytes to secrete key thymic factors, the researchers successfully restored T-cell populations and improved immune function in preclinical models. This mRNA-based intervention enabled the liver to act as a temporary source of signals normally provided by the thymus, enhancing T-cell survival, diversification, and functional competence. In aged mice, the approach improved responses to vaccination and potentiated the efficacy of cancer immunotherapy.
Published in Nature, these findings highlight a mechanistically innovative and translationally relevant approach to immune rejuvenation. By leveraging endogenous organ function to mimic thymic activity, this strategy offers a promising framework for sustaining adaptive immunity in aging populations, with potential applications in enhancing vaccine responsiveness, improving cancer immunotherapy outcomes, and mitigating age-related susceptibility to infectious disease.
Understanding Age-Related Immune Decline
T cells are central to adaptive immunity. They develop in the thymus, where immature progenitor cells undergo a rigorous selection process to ensure a diverse repertoire capable of recognizing and responding to a wide range of pathogens. The thymus also secretes cytokines and growth factors that promote T-cell survival. With age, the thymus progressively involutes, dramatically reducing the production of new T cells.
This decline has profound consequences: elderly individuals exhibit smaller, less diverse T-cell populations, respond more slowly to pathogens, and have diminished responses to vaccinations. Prior approaches to counteract thymic involution, including systemic administration of T-cell growth factors or transplantation of stem cells to regenerate thymic tissue have faced limitations. Systemic cytokine administration carries the risk of off-target effects and toxicity, while thymic transplantation or regeneration remains experimentally challenging.
The MIT team, led by Feng Zhang, James and Patricia Poitras Professor of Neuroscience, and co-lead author Mirco Friedrich, approached the problem differently. Instead of attempting to rebuild or replace the thymus, they sought to create a temporary, synthetic “factory” within the body capable of producing the T-cell-supporting signals that the aging thymus no longer generates.
Creating a Synthetic Factory in the Liver
The liver was selected as the site for this synthetic factory for several reasons. Hepatocytes, the primary liver cells, retain a robust capacity for protein synthesis even in advanced age. Additionally, the liver is highly accessible for mRNA delivery and receives the entirety of the body’s blood supply, including circulating T cells. This accessibility ensures that proteins produced by liver cells can quickly enter systemic circulation and exert their effects on the immune system.
The researchers identified three key thymic factors essential for T-cell survival and maturation: DLL1, FLT3L, and IL-7. They encoded the genes for these proteins into mRNA sequences and packaged them in lipid nanoparticles (LNPs), a delivery system that protects the mRNA and facilitates its uptake by hepatocytes. Once administered intravenously, the LNPs accumulated in the liver, where hepatocytes translated the mRNA into functional proteins. These proteins then circulated systemically, providing the same signals that a healthy thymus would normally supply.
This approach has several advantages over systemic cytokine delivery. By localizing production to the liver, protein levels can be more tightly controlled, reducing potential off-target effects. Moreover, the transient nature of mRNA ensures that the stimulation is temporary, minimizing the risk of overstimulation of the immune system.
Rejuvenating the Immune System in Aged Mice
The team conducted preclinical studies using 18-month-old mice, roughly equivalent to 50-year-old humans. Mice received multiple mRNA-LNP injections over four weeks to sustain production of the thymic factors. The results were striking:
Expansion of T-Cell Populations: Treated mice showed significantly larger and more diverse T-cell populations compared to untreated age-matched controls. Both CD4+ helper T cells and CD8+ cytotoxic T cells increased in number, indicating a broad rejuvenation of adaptive immunity.
Enhanced Response to Vaccination: The researchers tested the immune response using ovalbumin, a model antigen commonly used in immunological studies. Mice receiving the mRNA treatment prior to vaccination exhibited double the number of antigen-specific cytotoxic T cells compared to untreated mice, suggesting that the synthetic thymic signals effectively enhanced vaccine-induced immunity.
Improved Response to Cancer Immunotherapy: To explore therapeutic relevance, aged mice were implanted with tumors and treated with checkpoint inhibitors targeting PD-L1, which normally “release the brakes” on the immune system. Mice receiving the mRNA liver treatment showed higher survival rates and longer overall lifespan compared to mice receiving checkpoint inhibitors alone. This indicates that the approach can synergize with existing immunotherapies.
Critically, all three factors DLL1, FLT3L, and IL-7, were necessary to achieve the full rejuvenation effect. Administration of any single factor failed to produce the complete set of immune enhancements observed with the combined treatment.
A Synthetic, Transient, and Targeted Approach
Unlike attempts to regenerate or transplant thymic tissue, this strategy leverages the body’s existing organs to temporarily replicate essential thymic functions. Feng Zhang explains, “Our approach is more of a synthetic approach. We’re engineering the body to mimic thymic factor secretion.” The transient nature of mRNA-mediated protein expression ensures safety and flexibility, while targeting the liver maximizes protein output and systemic distribution.
By converting the liver into a temporary factory, researchers circumvent the challenges of thymic involution without the need for permanent organ replacement. Moreover, this platform could, in principle, be adapted to deliver additional immune factors, offering a flexible system for rejuvenating immunity in different contexts, including infections, vaccination, and cancer treatment.
Implications for Human Health
If successfully translated into humans, this approach could have profound implications:
Improved Vaccine Efficacy in Older Adults: Age-related declines in T-cell populations contribute to weaker responses to vaccines. Rejuvenating T-cell populations prior to vaccination could increase the effectiveness of seasonal influenza, COVID-19, and other immunizations.
Enhanced Cancer Immunotherapy: Older patients often respond less effectively to checkpoint inhibitors and other immunotherapies. By increasing the size and diversity of T-cell populations, this strategy may improve therapeutic outcomes.
General Immune Rejuvenation: Beyond vaccination and cancer therapy, boosting T-cell populations could reduce susceptibility to infections in the elderly and improve overall immune resilience.
Friedrich notes, “As we get older, the immune system begins to decline. We wanted to think about how we can maintain this kind of immune protection for a longer period of time, and that’s what led us to think about how to boost immunity.”
Future Directions
The researchers plan to expand studies to other animal models and explore additional thymic factors that may further enhance immune function. They are also interested in assessing effects on other immune cell populations, including B cells, which are critical for antibody production.
Long-term, the goal is to develop interventions that could safely enhance immune function in humans, potentially delaying or reversing aspects of immunosenescence. The research was funded in part by the Howard Hughes Medical Institute, the K. Lisa Yang Brain-Body Center, Broad Institute Programmable Therapeutics Gift Donors, the Pershing Square Foundation, and other foundations supporting translational science.
Conclusion
This study represents a paradigm shift in how researchers approach immune rejuvenation. By leveraging mRNA technology to convert the liver into a temporary thymic factor factory, MIT and Broad Institute scientists have demonstrated a method to restore T-cell populations, enhance vaccine responses, and improve cancer immunotherapy outcomes in aged organisms. This synthetic, transient, and targeted approach could pave the way for therapies that maintain immune competence throughout aging, offering the potential for healthier and longer lives.
As Zhang notes, “If we can restore something essential like the immune system, hopefully we can help people stay free of disease for a longer span of their life.” The study not only deepens our understanding of immune system aging but also provides a promising framework for translational interventions to enhance human health.