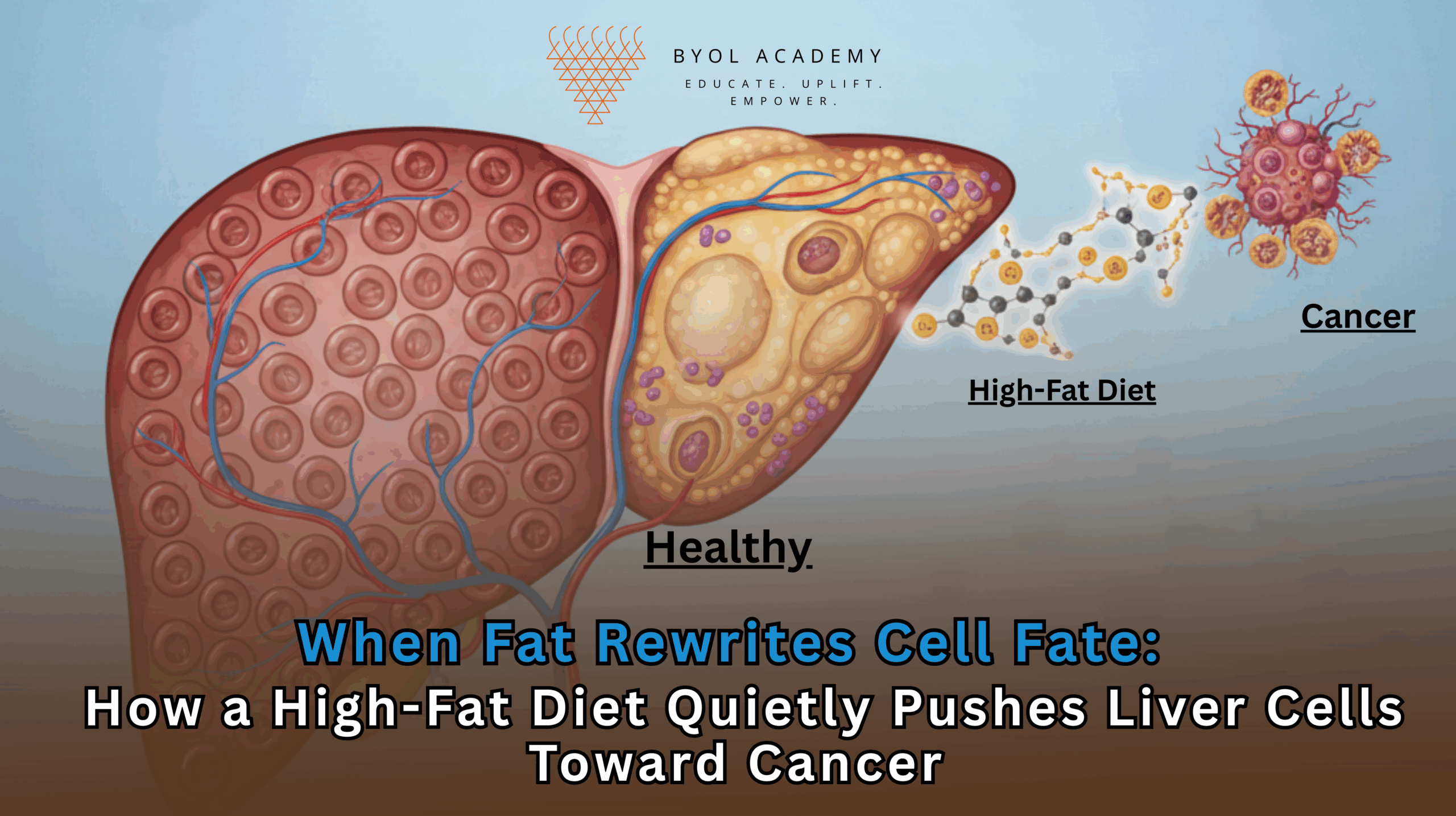Liver cancer is one of the fastest-rising causes of cancer-related deaths worldwide, closely tracking the global surge in obesity, metabolic […]
IL-36 Gamma-Enhanced CAR T Cells Offer New Therapeutic Avenue in Solid Tumors
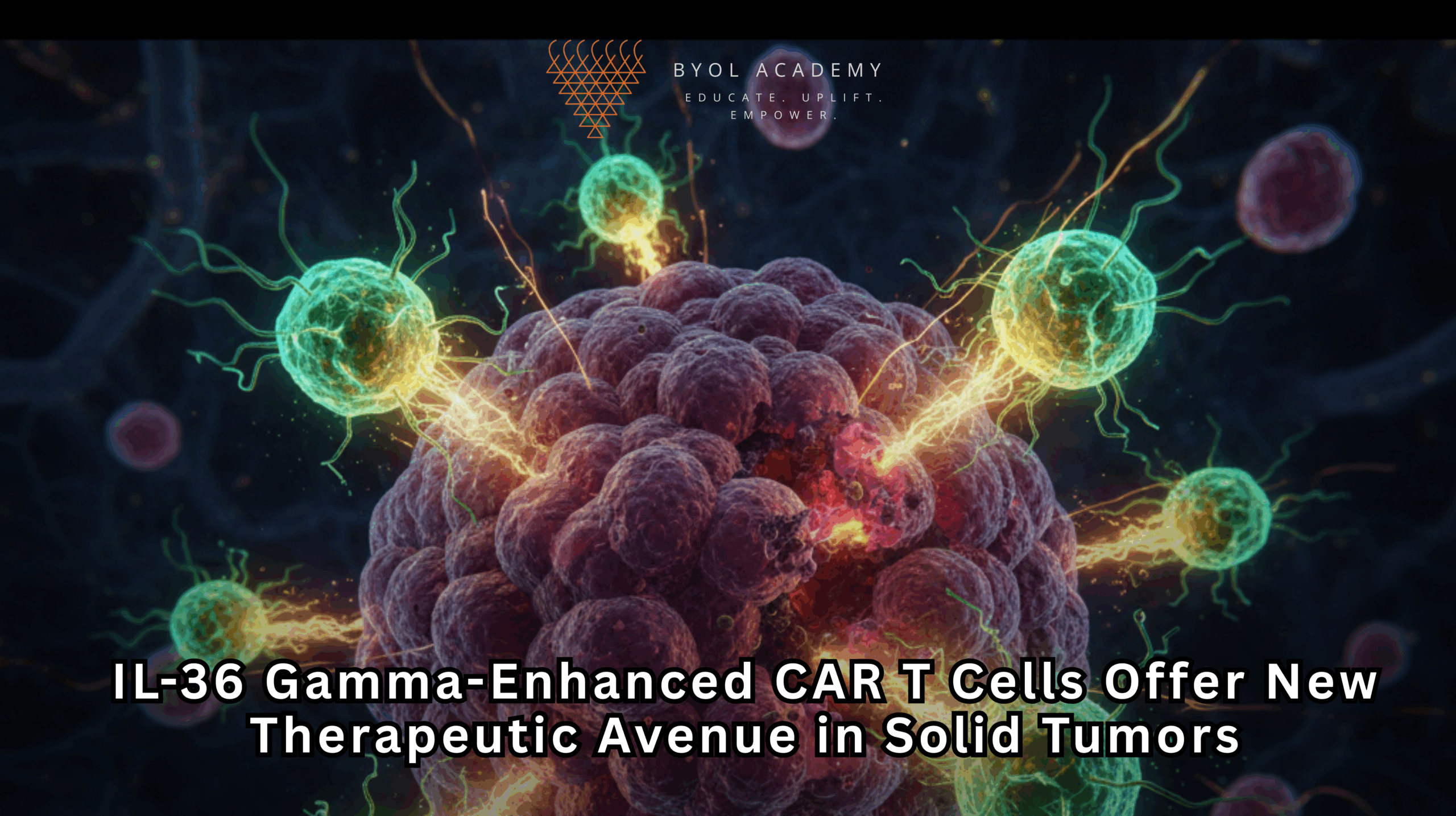
Chimeric antigen receptor (CAR) T-cell therapy has revolutionized the management of hematologic malignancies, achieving unprecedented response rates in B-cell acute lymphoblastic leukemia and diffuse large B-cell lymphoma. Despite these advances, CAR T-cell therapy for solid tumors has historically been ineffective due to several inherent obstacles: heterogeneous antigen expression, the immunosuppressive tumor microenvironment (TME), and physical barriers such as dense extracellular matrices and abnormal vasculature that prevent T-cell infiltration. Solid tumors are also capable of antigen downregulation or loss, allowing tumor escape from targeted immunotherapy. These factors collectively have limited clinical efficacy and presented a long-standing challenge in oncology.
IL‑36γ-armored CAR T cells represent a next-generation approach that addresses these challenges. By arming CAR T cells with the pro-inflammatory cytokine IL‑36γ, this platform not only exerts direct cytotoxicity against tumor cells but also recruits and reprograms host immune cells, including neutrophils and endogenous T cells, to establish a multi-pronged antitumor response. This dual mechanism potentially overcomes key resistance mechanisms inherent to solid tumors.
Mechanistic Rationale
IL‑36γ, a member of the IL-1 cytokine family, is known to modulate both innate and adaptive immunity. In the engineered CAR T-cell platform, IL‑36γ is constitutively secreted by the CAR T cells upon tumor engagement. This local cytokine release exerts multiple effects:
1. Reprogramming neutrophils within the tumor microenvironment to adopt antigen-presenting functions, including upregulation of MHC-II and co-stimulatory molecules such as CD80 and CD86.
2. Recruiting and activating endogenous T cells, facilitating epitope spreading and broadening antitumor immunity beyond CAR-specific targets.
3. Remodeling the tumor microenvironment, reducing immunosuppressive populations such as regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), and enhancing chemokine gradients that promote CAR T-cell infiltration.
This multi-faceted mechanism allows IL‑36γ-armored CAR T cells to target tumors with heterogeneous antigen expression, resist immune suppression, and maintain long-term efficacy.
Preclinical Study Design and Methodology
The study utilized murine xenograft models of small cell lung carcinoma to evaluate the efficacy of IL‑36γ-armored CAR T cells. Key elements of the experimental design included:
- Tumor Establishment: Immunodeficient mice were implanted subcutaneously with human small cell lung carcinoma cells to generate established solid tumors.
- CAR T-Cell Engineering: Human T cells were genetically modified to express a CAR targeting tumor antigens and constitutively secrete IL‑36γ.
- Treatment Protocol: CAR T cells were administered intravenously without prior lymphodepletion, a departure from conventional protocols that aim to eliminate endogenous lymphocytes.
- Assessment of Tumor Response: Tumor growth was monitored through caliper measurements, bioluminescence imaging, and histopathological analyses.
- Immune Profiling: Tumor-infiltrating leukocytes were analyzed via flow cytometry, immunohistochemistry, and single-cell RNA sequencing to evaluate neutrophil reprogramming, T-cell activation, and TME remodeling.
Key Preclinical Findings
IL‑36γ-armored CAR T cells demonstrated complete tumor eradication in all treated mice, whereas conventional CAR T-cell therapy achieved only partial or transient responses. Detailed analyses revealed:
- Neutrophil Reprogramming: Tumor-associated neutrophils upregulated MHC-II and co-stimulatory molecules, functioning as antigen-presenting cells to prime endogenous T cells.
- Enhanced T-Cell Infiltration: CD8+ T-cell numbers within the tumor increased significantly, accompanied by a reduction in Tregs and MDSCs.
- TME Remodeling: The cytokine-rich environment altered chemokine profiles and reduced immunosuppressive signaling, supporting CAR T-cell expansion and persistence.
- No Lymphodepletion Required: Preservation of host neutrophils allowed active participation in antitumor responses, minimizing toxicity associated with preconditioning chemotherapy.
- Durable Antitumor Immunity: Treated mice exhibited long-term protection against tumor rechallenge, indicating induction of immune memory.
These findings establish that IL‑36γ-armored CAR T cells leverage both direct cytotoxicity and host immune collaboration, addressing major hurdles of conventional CAR T-cell therapy in solid tumors.
Mechanistic Insights
The study elucidates several novel immunologic mechanisms:
- Antigen-Presenting Neutrophils: Neutrophils, traditionally considered terminal effector cells, acquire antigen-presenting capabilities, priming naïve T cells and enhancing epitope spreading.
- Endogenous Immune Activation: Recruited T cells amplify the antitumor response beyond the CAR T-cell specificity, providing broader coverage against heterogeneous tumor antigens.
- Microenvironment Modulation: IL‑36γ signaling diminishes immunosuppressive populations and enhances chemotaxis of effector cells, facilitating sustained CAR T-cell activity within tumors.
This integrated mechanism represents a paradigm shift in solid tumor immunotherapy, combining cellular engineering with immunomodulation to overcome barriers that have historically limited CAR T efficacy.
Clinical Implications
IL‑36γ-armored CAR T cells offer several potential advantages for future clinical translation:
1. Therapeutic Potential in Refractory Solid Tumors: Preclinical success supports evaluation in small cell lung carcinoma, pancreatic adenocarcinoma, and other refractory solid tumors.
2. Reduced Treatment Toxicity: Avoiding lymphodepletion decreases the risk of chemotherapy-associated adverse events.
3. Broad and Durable Immunity: Recruitment of host immune cells may lead to sustained antitumor immunity and reduced risk of relapse.
4. Integration with Combination Therapies: Potential exists for combining IL‑36γ-armored CAR T cells with checkpoint inhibitors or other immunomodulatory agents to enhance therapeutic outcomes.
Challenges and Considerations
Despite the promising results, several translational challenges remain:
- Safety Monitoring: IL‑36γ secretion may trigger cytokine release syndrome or local inflammatory reactions. Careful dose optimization and monitoring protocols are essential.
- Manufacturing Complexity: Engineering CAR T cells to co-express cytokines requires advanced GMP-compliant manufacturing processes.
- Tumor Heterogeneity: Efficacy must be validated across diverse tumor types with varying antigen profiles.
- Regulatory Considerations: Early engagement with the FDA and EMA is critical for clinical trial design, safety assessment, and approval pathways.
Future Directions
The research team outlines a roadmap for clinical translation:
Phase I Clinical Trials: Focused on safety, tolerability, and preliminary efficacy in patients with advanced solid tumors.
Combination Immunotherapy Studies: Evaluating synergy with checkpoint inhibitors, targeted therapies, or TME-modulating agents.
Biomarker Development: Identification of predictive markers to select patients most likely to respond to therapy.
Long-Term Immune Memory: Assessing durability of antitumor responses and protection against tumor relapse.
Expansion Across Tumor Types: Testing efficacy in pancreatic, colorectal, and neuroendocrine cancers to broaden therapeutic applicability.
Conclusion
IL‑36γ-armored CAR T cells represent a next-generation immunotherapy platform for solid tumors. By engaging both innate and adaptive immune systems, reprogramming neutrophils, and remodeling the tumor microenvironment, these engineered cells achieve complete tumor eradication in preclinical models without the need for lymphodepletion. This approach addresses the major limitations of conventional CAR T-cell therapy, including antigen heterogeneity and TME-mediated immunosuppression, and provides a strong rationale for early-phase clinical trials. IL‑36γ-armored CAR T cells have the potential to transform the therapeutic landscape for refractory solid tumors, offering durable and broad antitumor immunity.