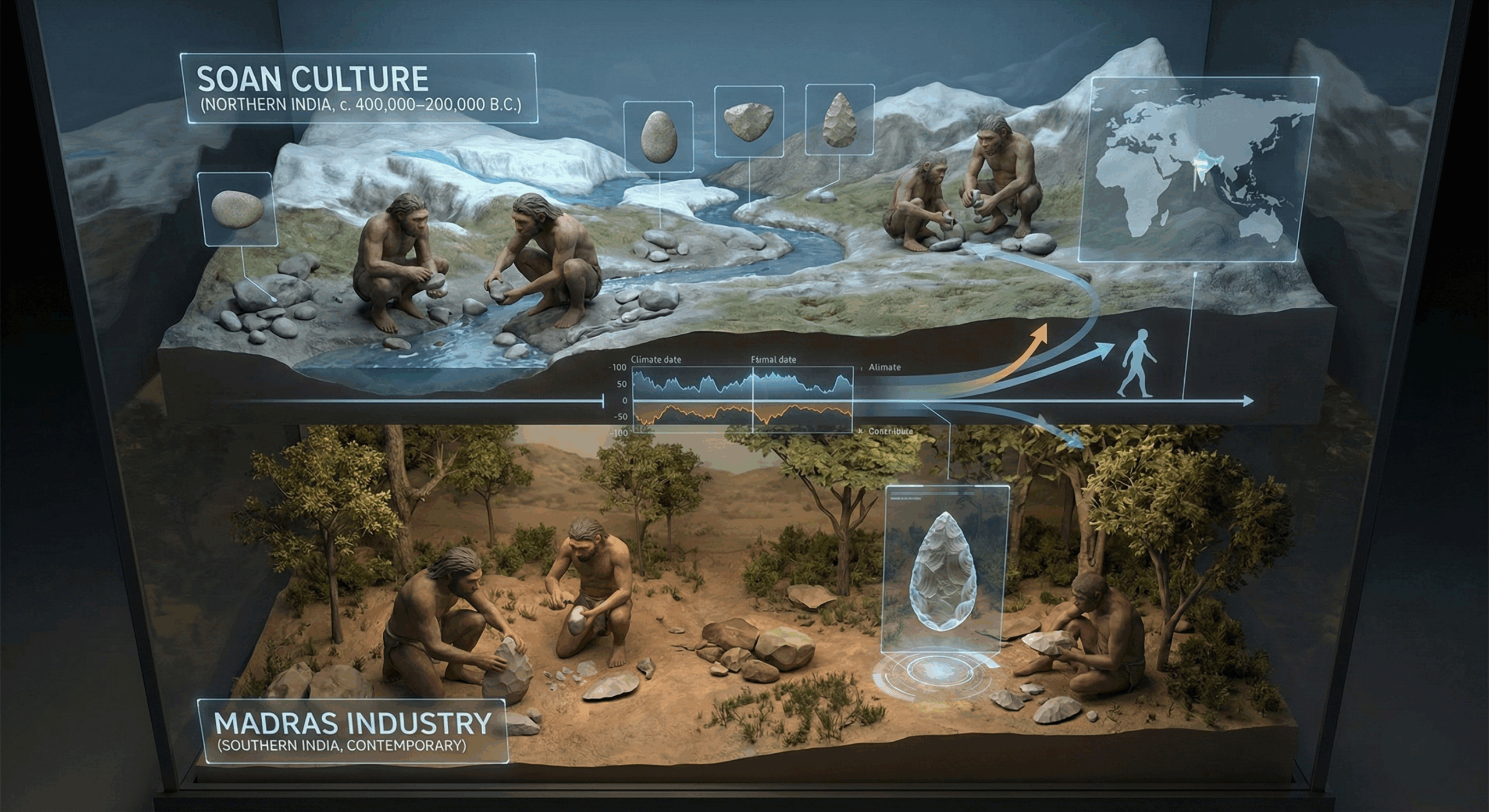
The Soan and Madras industry related to stone age times in Indian sub-continent.
Golden Blood Type (Rh-null): The World’s Rarest Blood Phenotype

Overview
The Rh-null or “golden blood” phenotype is an extraordinary immunohematological condition characterized by the complete absence of all Rh system antigens from erythrocyte membranes. With fewer than 50 known individuals worldwide, Rh-null remains the rarest and most valuable blood type known to medicine. Its immunological universality within the Rh system makes it a potential lifesaver for patients with complex Rh antigen profiles, while its scarcity presents significant transfusion challenges. This review discusses the genetic etiology, biochemical features, clinical manifestations, and global transfusion implications of the Rh-null phenotype, emphasizing its scientific and humanitarian significance.
Introduction
Blood group systems form the foundation of transfusion medicine, with the ABO and Rh systems being the most clinically significant. The Rh system, comprising over 60 antigens, plays a crucial role in hemolytic disease, transfusion compatibility, and erythrocyte membrane integrity.
Among these, the Rh-null phenotype represents the complete deletion of all Rh antigens — including D, C, c, E, e, and others — on the red blood cell surface. First described in 1961 in an Aboriginal Australian woman, this discovery transformed understanding of Rh antigen biology and membrane physiology. Due to its extreme rarity and scientific value, Rh-null has been termed “golden blood.”
Epidemiology and Global Occurrence
Rh-null blood is estimated to occur in approximately 1 per 6 million individuals. To date, fewer than 50 confirmed cases exist globally.
These individuals belong to various ethnic backgrounds, confirming that the phenotype is not ethnicity-restricted. The International Rare Donor Panel (IRDP) maintains registry coordination to facilitate Rh-null transfusion requests worldwide, emphasizing the need for global donor surveillance and cryogenic storage.
Genetic and Molecular Basis
The Rh-null phenotype arises through two principal genetic mechanisms:
- Regulator Type Rh-null:
Caused by mutations in the RHAG gene (Rh-associated glycoprotein), responsible for the assembly and transport of Rh proteins to the erythrocyte membrane. - Amorph Type Rh-null:
Results from inactivating mutations in both RHD and RHCE genes, leading to the absence of Rh structural proteins.
Loss of RHAG function disrupts erythrocyte membrane stability, altering cation transport and increasing osmotic fragility. This defect also provides key insights into the functional role of Rh proteins in gas transport and cellular homeostasis.
Clinical and Hematological Features
While some Rh-null individuals remain asymptomatic, others exhibit hematological and morphological abnormalities due to altered membrane architecture:
- Mild to moderate chronic hemolytic anemia
- Spherocytosis or stomatocytosis of red blood cells
- Decreased red cell lifespan and increased osmotic fragility
- Jaundice in severe hemolysis cases
- Pregnancy complications due to Rh isoimmunization if carrying Rh-positive fetus
During systemic infections, hemolytic crises can occur, occasionally progressing to acute kidney injury due to free hemoglobin deposition.
Transfusion Medicine and Compatibility
Rh-null blood is considered the universal donor for patients with rare Rh variants because of its complete antigenic neutrality. However, the recipients with Rh-null blood face life-threatening challenges — they can only receive Rh-null blood.
This duality makes Rh-null both medically priceless and clinically precarious.
Key Transfusion Challenges
- Limited donor pool: <10 active donors globally.
- International coordination required for blood transport via ISBT networks.
- Cryopreservation at -80°C or in liquid nitrogen is standard for emergency use.
- Ethical dilemmas: balancing research value versus patient needs.
Comparison with Other Rare Blood Types
Blood Phenotype Approximate Frequency Key Features / Clinical Note
- Rh-null (Golden Blood) < 50 known cases Absence of all Rh antigens; universal donor (within Rh)
- Bombay (hh) phenotype ~1 in 4 million Lacks H antigen (foundation for ABO), extremely limited compatibility
- AB negative (AB–) ~0.5–1% of population Rare among common ABO types, but manageable in modern transfusion systems
Compared to other rare phenotypes, Rh-null remains unparalleled in its antigenic absence and resultant challenges.
Advances, Research & Future Directions
Genetic & Functional Studies
- The 2025 novel mutation study enhances understanding of how small genetic defects can cascade to complete Rh antigen absence.
- Rh-null cells are also used as model systems to study erythrocyte membrane transport, gas diffusion (e.g. CO₂, NH₃), and protein complex assembly.
Biobanking & Cryotechniques
- Enhanced cryopreservation protocols and better maintenance of rare blood units are under development to optimize cell viability after thawing.
Registry Expansion & AI Support
- Growing global networks are expanding their rare donor registries. Emerging approaches, such as AI-driven matching algorithms, may help expedite locating compatible units across continents.
Gene Therapy Possibilities
- Though speculative, future gene editing or cell therapy approaches might one day enable in vivo correction or reconstitution of lacking Rh proteins — a tantalizing concept for personalized medicine.
Research and Biomedical Importance
Rh-null blood has become a cornerstone for molecular hematology and membrane biology research. Studies using Rh-null erythrocytes have provided insights into:
- Erythrocyte membrane transport functions (CO₂ and NH₃ diffusion).
- Rh protein complex assembly mechanisms.
- Evolutionary aspects of human blood group polymorphism.
- Potential development of synthetic blood substitutes mimicking antigen-free profiles.
Thus, Rh-null blood is not merely a medical rarity but a biological model system for studying red cell physiology and antigen expression.
Ethical, Logistical & Policy Considerations
- Donor consent & confidentiality: Because donors are extremely few and identifiable, robust protections are needed.
- Allocation policies: Prioritizing life-saving transfusions over research use is essential.
- Cost & transport infrastructure: Ensuring equitable access to Rh-null units in remote or underserved regions remains a major barrier.
- Regulatory harmonization: International frameworks (e.g. WHO, ISBT) must align licensing, cross-border couriering, cold-chain standards, and liability rules.
Conclusion
The Rh-null (“golden blood”) phenotype epitomizes the intersection of rarity, scientific intrigue, and humanitarian importance in modern transfusion medicine. While its universal compatibility within the Rh system offers unparalleled therapeutic potential, its scarcity imposes immense logistical and ethical challenges.
Ongoing genetic research, enhanced rare donor registries, and advanced preservation technologies are imperative to ensure that this “golden” gift of nature continues to illuminate the frontiers of hematology.
References
- MedicineNet. What Is the Golden Blood Type? 2025. Available from: https://www.medicinenet.com/what_is_the_golden_blood_type/article.htm
- Daniels G. Human Blood Groups. 3rd ed. Wiley-Blackwell; 2013.
- Reid ME, Lomas-Francis C, Olsson ML. The Blood Group Antigen FactsBook. 4th ed. Academic Press; 2024.
- International Society of Blood Transfusion (ISBT). Rare Donor Program and Rh System Classification. Geneva: ISBT; 2025.
- Flegel WA. Molecular genetics and clinical relevance of the Rh blood group system. Blood Rev. 2020;44:100678.
- Medical News Today. Rarest Blood Type: Chart and Compatibility (Updated 2025).
- Blood Matters / Our Blood Institute. The World’s Rarest Blood Type Is Rhnull. 2025.
- Pande BS. Rare Blood Group: A Golden Blood. Asian J Nursing Education & Research. 2023.